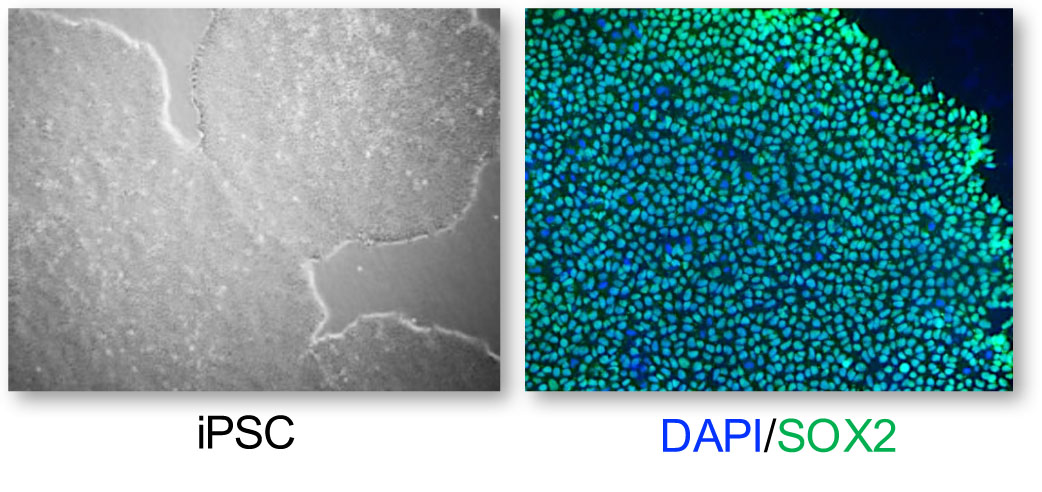
Given the complexity and heterogeneity of IRDs, it remains essential to understand the pathophysiological mechanisms underlying these dystrophies and to develop therapies to treat them. Our main, but not exclusive, working model is the human retinal model derived from induced pluripotent stem cells (iPSCs) that we generate from skin biopsies of patients within the framework of our national reference centre, Maolya.
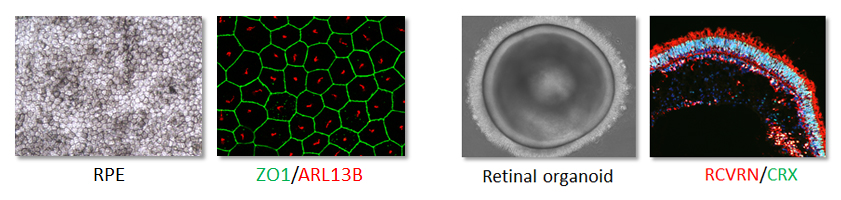
We differentiate iPSCs into either retinal pigment epithelium or 3D retinal organoids containing photoreceptors. These models allow us to replicate the retina of patients in a culture dish to study dysfunctional processes.
We also use these models to evaluate the efficacy of personalized therapies, such as gene therapy (gene replacement, genome editing, antisense oligonucleotides etc.), on the tissue truly affected by the disease. The retina is a perfect target for gene-based therapeutic approaches because it is easily accessible through minimally invasive methods, it has a small surface area allowing the use of low vector doses, and it is immune-privileged (isolated from the immune system) thanks to the blood-retinal barrier. Furthermore, IRDs are monogenic (only one gene needs to be supplemented or targeted) and can be diagnosed early in the course of the disease by specific clinical signs. Moreover, they progress relatively slowly towards blindness, thus providing a wide therapeutic window. We are currently working on several genes responsible for IRDs such as CHM, RLBP1, USH2A, NR2E3, ABCA4, and CRX.
Our work aims to generate and use retinal cell models of IRDs to verify the effect of genetic variants and/or validate novel causative genes, elucidate the pathophysiological mechanisms, and develop innovative therapies. This "patient-to-patient" approach allows us to return to the patients by offering them better care or a novel therapeutic approach.
Recent publications
Bocquet et al., JCI Insight, 8: e169426, 2023
Sanjurjo-Soriano et al., HGG Adv, 4: 100229, 2023
Sanjurjo-Soriano et al., Methods Mol Biol, 2454: 589-606, 2022
Sanjurjo-Soriano et al., Stem Cell Res, 60: 102378, 2022
Sanjurjo-Soriano et al., Stem Cell Res Ther, 13: 1508, 2022
Olivier et al., Genes, 13: 1508, 2022
Jazouli et al., Adv Stem Cell Biol, 15: 123-145, 2022
Vincent et al., Pharmaceutics, 14:1029, 2022
Kalatzis et al., Mol Diagn Ther, 25: 661-675, 2021
Mamaeva et al., FASEB J, 35: e21406, 2021
Diakatou et al., Int J Mol Sci, 22: 2607, 2021
Moine et al., Free Radic Biol Med, 162: 367-382, 2021
Collaborations
Pr. Ian MacDonald, Alberta University, Edmonton, Canada
Pr. Elfride de Baere, Ghent University, Ghent, Belgium
Dr. Erwin Van Wijk, Radboud University Medical Centre, Nijmegen, Netherlands
Dr Kerstin Nagel-Wolfrum, Johanes Gutenberg University of Mainz, Mainz, Germany
Pr. Katrien Reamut, University of Ghent, Ghent, Belgium
Dr. Bence Gygory, Institute of Molecular and Clinical Ophthalmology, Basel, Switzerland
Dr. Susanne Kohl, University of Tübingen, Tübingen, Germany
Pr. Hélène Dollfus, University Hospital of Strasbourg, Strasbourg France
Member of the European Retinal Disease Consortium