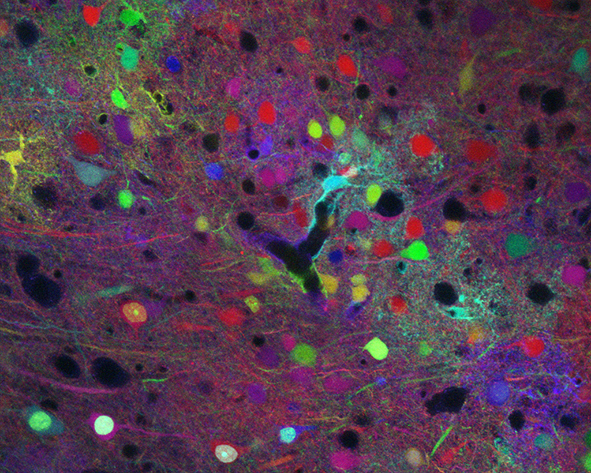
The mammalian brain relies on many distinct cell types (including neurons and macroglial cells such as astrocytes and oligodendrocytes) to exert its high cognitive functions. The cerebral cortex is composed of projection (or excitatory/pyramidal) and local circuit neurons (or inhibitory/interneurons) deployed in six horizontal layers. Since a first description as « nerve glue » suggesting a homogeneous population of neuron supporting cells, tremendous progress had been made to uncover numerous active contributions of mature glia, and more particularly of astrocytes, its most abundant representative, to metabolite support, synapse function and neurovascular modulation. Oligodendrocytes ensheath axons enabling rapid saltatory conduction and provide metabolic support to neurons. During the past decades, our knowledge on cortical development and associated pathologies has been greatly improved by studies focusing on a single cell type (neurons, astrocytes or oligodendrocytes).
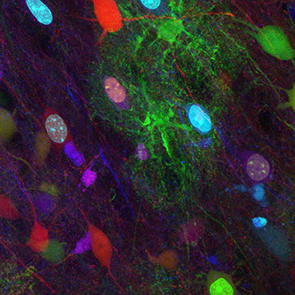
Although these works have provided a better knowledge of the contribution of each cell type to brain maturation and function, we still do not fully apprehend the extent of cerebral cortex diversity and the cellular and molecular mechanisms involved in the sequential production and subsequent partnership of distinct cell types in healthy and diseased brain.
To shape an operative brain, neuronal and glial cells must be produced in defined proportions and locations following a strict spatiotemporal regulation whose imbalance may lead to dramatic brain disorders. How neural diversity arises from seemingly homogeneous population of neural progenitors and how the balance between neuronal and glial cell types is achieved and regulated during development remain challenging questions not yet addressed in vivo.
Our work aims at providing answers to the following questions:
- How is neuronal and glial partnership built during corticogenesis and what are the key regulators of this crosstalk?
- What is the extent of neural diversity generated from cortical progenitors?
- How are neuron-glial networks and cell diversity affected in neuropsychiatric disorders?
To answer these questions, our team takes advantages of genetic engineering (including MAGIC Markers strategies), animal models, histological and multicolor imaging (confocal and biphoton) approaches.
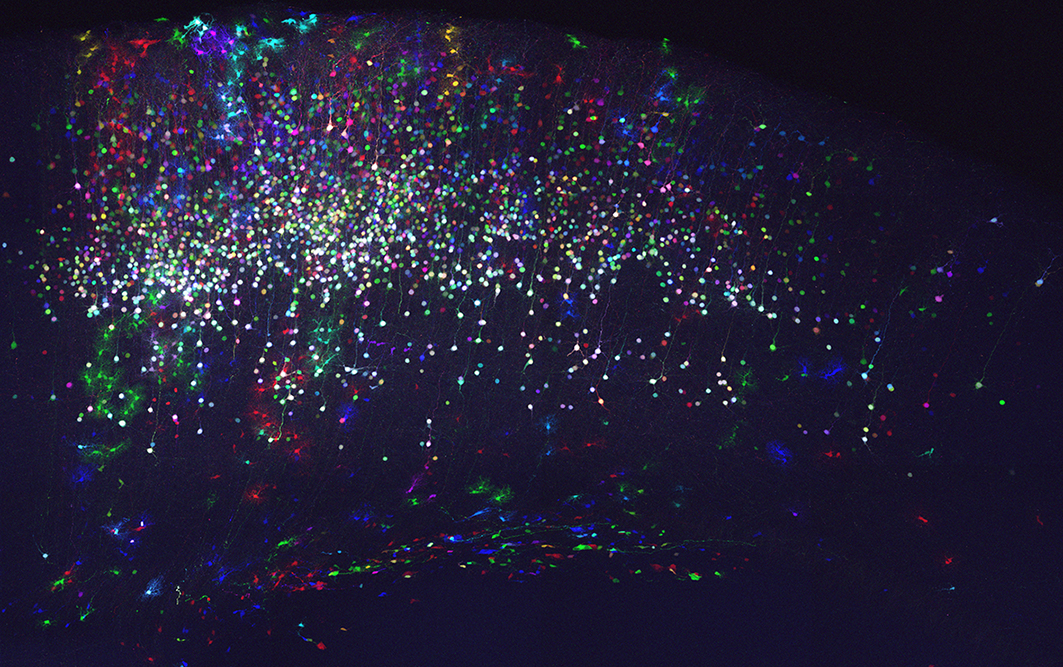
http://www.corticogenesislab.toile-libre.org/EN_main_page.html
Major publications
Guesmi K. et al., Light: Science & Applications, 2018
Saadaoui M. et al., EMBO Reports, 2017
Loulier K. et al, Neuron, 2014
Tabansky I. et al., Curr Biol, 2013
Mahou P. et al., Nat Methods, 2012
Loulier K. et al., PLoS Biology, 2009
Angot E. et al., Stem Cells, 2008
Loulier K. et al., J. Neurochem, 2006
Rossel M. et al., Development, 2005
Hack I. et al., Nat. Neurosci, 2002
Collaborations
- Emmanuel Beaurepaire, LOB, Ecole Polytechnique, Palaiseau
- Martine Cohen-Salmon, Collège de France, Paris
- Harold Cremer, IBDML, Marseille
- Véronique Perrier, MMDN, Montpellier
- Jean Livet, Institut de la Vision, Paris
- Xavier Nicol, Institut de la Vision, Paris
Fundings




Contact