Our interest
Most of our organs are constantly renewed through the involvement of stem cell population(s). These stem cells are strictly regulated to ensure a life-long homeostasis. The fine regulation of cell fate applied on stem cells to progenitors and finally terminally differentiated cells is instrumental to the organ function. Because of aging, disease, or injury, the organ homeostasis collapses, leading to a transitory or permanent organ failure. To restore the homeostasis, or to replace the whole organ, the tissue-specific cell fate needs to be well known at the molecular and population level.
Our aspiration is to decipher the molecular networks involved in cell fate acquisition, maintenance and differentiation to propose new regenerative medicine strategies.
Our model
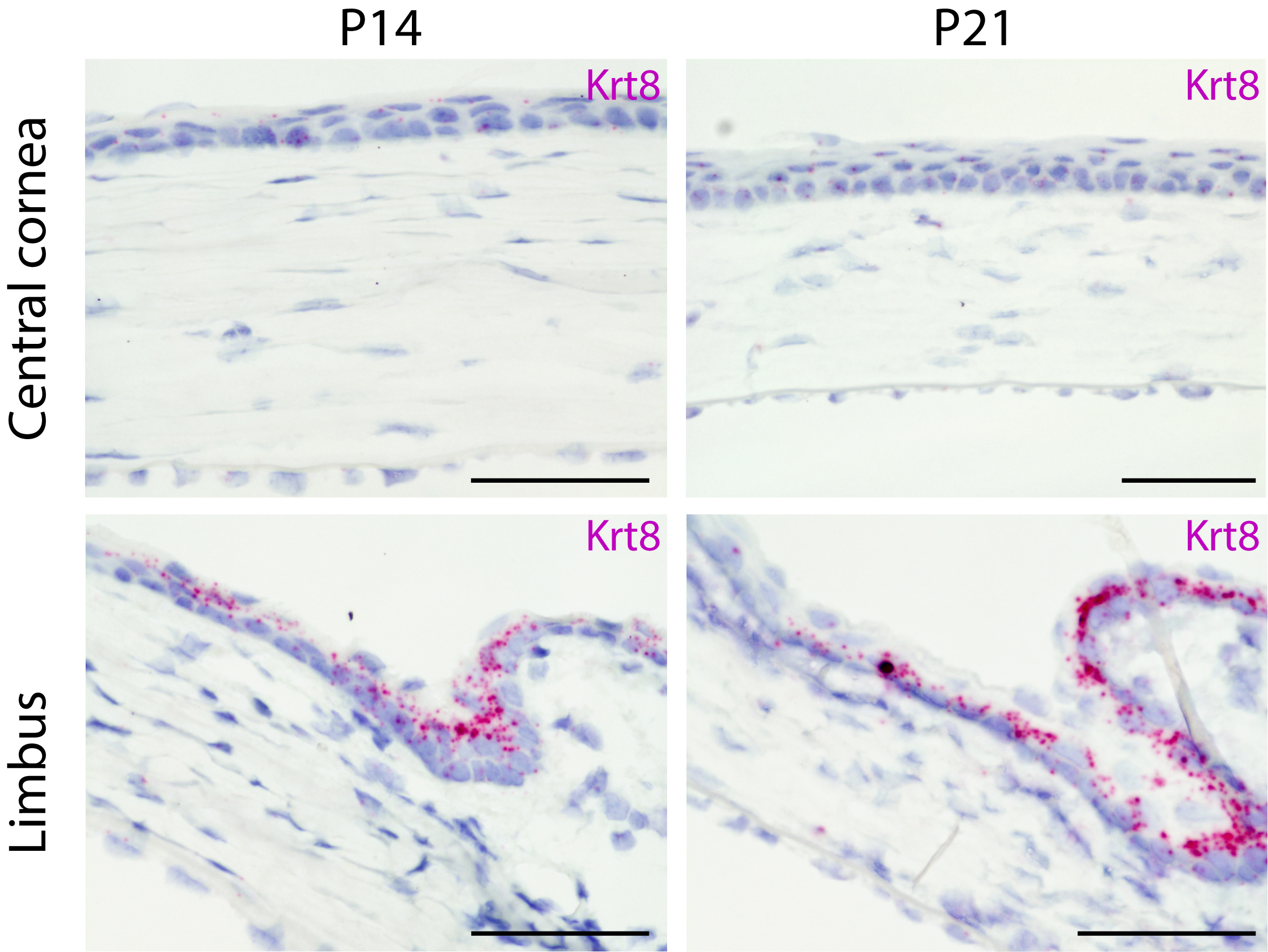
The cornea is the transparent tissue at the surface of the eye. It is composed of a stratified epithelium supported by a mesenchymal stroma. This epithelial compartment undergoes a life-long renewal, which is instrumental to maintain a clear vision. The main stem cell niche is localized in the limbus that forms the border between the avascularised cornea and the vascularised conjunctiva.
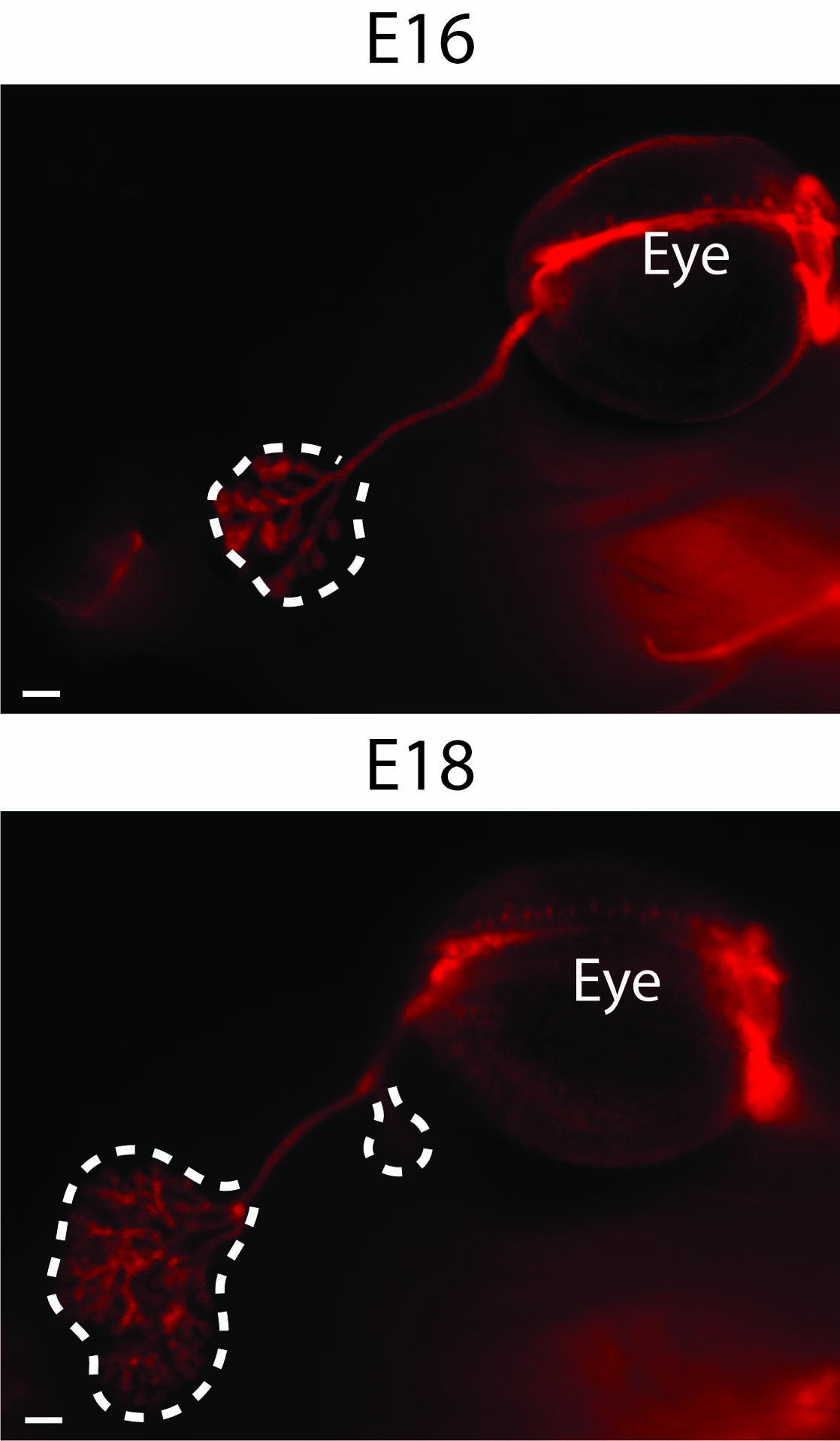
Interestingly, the cornea is the most densely innervated tissue in the body. The sensory neurons, originating from the trigeminal ganglia, are infiltrating the epithelium during cornea morphogenesis, and will be gradually lost upon aging.
Injuries, aging, or diseases can lead to a loss of cornea transparency, and ultimately loss of vision. Around 28 million people suffer from corneal defects worldwide, among them 10 million people are corneal blind. The most prominent causes are Dry Eye Diseases (up to 34% of the elderly), and corneal abrasions (about 1 million cases per year in USA alone). Unfortunately, the lack of cornea donors is a roadblock to treat all patients.
Stem-ming knowledge for regenerative medicine
The cornea homeostasis defects, common in an aging population, combined to the incidence of corneal insults, support the urgency of developing new research avenues.
We focus on the steps leading the stem cell fate determination and on the molecular cues that trigger their homing to the niche. Moreover, we decipher the genetic network, which either maintains the stemness or leads to the cell differentiation, and how this network is modulated during aging and/or upon corneal trauma.
Major Publications
Kalha S., et al., J Vis Exp 10:137. 2018
Kalha S., et al., Stem Cells 36:562-73. 2018
Sanz Navarro M., et al., Development 145. 2017
Kuony A., and Michon F. Front Physiol 8:739. 2017
Sun Z., et al. Development 143: 4115-26. 2016
Yang Z., et al., Stem Cells. 33: 1670-81. 2015
Stratoulias V., et al., PLoS One 9: e101141. 2014
Collaborations
Fundings

![]()
Contact