Although a variety of primary sensory receptor neurons and spinal interneurons are implicated in the detection and transmission of different sensory modalities, how they arise during development remains poorly understood. The process of neuronal specification is the acquisition of definitive phenotypic characteristics for a given subclass of neurons during embryonic development. This acquisition can be divided into several interdependent and sequential phases, from the time point when progenitor cells exit the cell cycle toward the newly formed and perfectly differentiated neuron gains its definitive physiological function, passing through phases of specific biochemical characteristics acquisition, axonal growth, survival, set-up and maintenance of connectivity with the appropriate targets.
Depending on their functional specificities, different DRG neuron sub-types display great morphological, molecular and electrophysiological diversity. Similarly, the mechanisms regulating the precise connectivity essential for the formation of spinal circuits transducing sensory information are not yet elucidated.
i) Specification of somatosensory neuron sub-types: We use neuroanatomical, electrophysiological and behavioural studies analyses on transgenic mice models to study the roles of members of the Maf, Zeb, Neurogenin and Meis transcription factor families that are expressed in sub-types of somatosensory precursors.
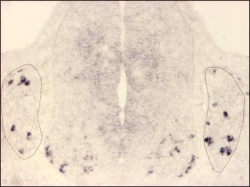
MafA expression detected by in situ hybridisation on a cross-section of an E11.5 mouse embryo.
ii) Activity dependent mechanisms refine synaptic connections during critical periods of embryonic and early postnatal development. The spinal nociceptive system, however, undergoes refinement and maturation despite the rare occurrence of noxious or harmful stimuli during early development. In addition to their roles in tactile sensation, input from Abeta afferent low threshold mechanoreceptor (LTMRs) neurons to the spinal cord is thought to be important for the postnatal refinement of nociceptive circuits and, at adult stages, for driving nerve injury induced neuropathic pain. However, exactly how Abeta fibre input drives these processes is not well-known. We use mouse genetic tools that allow the specific ablation or activity modulation of LTMRs in a temporally-controlled way. The functional consequences of LTMR modification at different developmental time-points are studied in collaboration with our partner teams specialized in electrophysiological analyses.
Major publications
Venteo S. et al., Cell Rep. 29(10):2953-2960, 2019
Babski H. et al., Nat Commun. 22;10(1):4796, 2019
Ohayon D., et al., Cell Rep. 17:1473-1481, 2016
Bouilloux F., et al., Elife. Feb 8;5. pii: e11627, 2016
Ohayon D., et al., Dev Cell 33:343-50, 2015
Wende H., et al., Science 335(6074):1373-6, 2016
Grimal S., et al., Glia 58(16):1977-87, 2010
Bourane S.*, Garces A.*, et al., Neuron 64(6):857-70, 2009
Ohayon D.*, Pattyn A.*, et al., EMBO J. 28(20):3228-43, 2009
Collaborations
- Goulding, Salk Institut, San Diego
- Dallel / C. Peirs, Neuro-Dol, Clermont-Ferrand
- Lewin, Max Delbrück center, Berlin
- Lallemend, Karolinska institutet, Stockholm
Fundings