Stress has been labelled by the W.H.O as the 21st century worldwide health epidemic. Stress, especially during critical periods of development, can result in long-term alterations in brain structures and stress signalling. Individual differences in fear learning/extinction and information processing of traumatic memories could differentially contribute to the onset of neurocognitive disorders, further modulated by resilience capacity. Our purpose is to determine the relative contributions and interaction of biological, behavioural and environmental factors to the loss (or preservation) of cognitive functions with ageing, with a particular focus on stress-related neuromarkers. We hypothesize that severe life events can lead to persistent dysregulation of stress systems, increasing vulnerability to neuropsychiatric disorders in adults and neurodegenerative disorders in later-life. The unifying hypothesis of the projects undertaken by our group is that biomarkers of stress exposure may constitute robust predictors of disease risk.
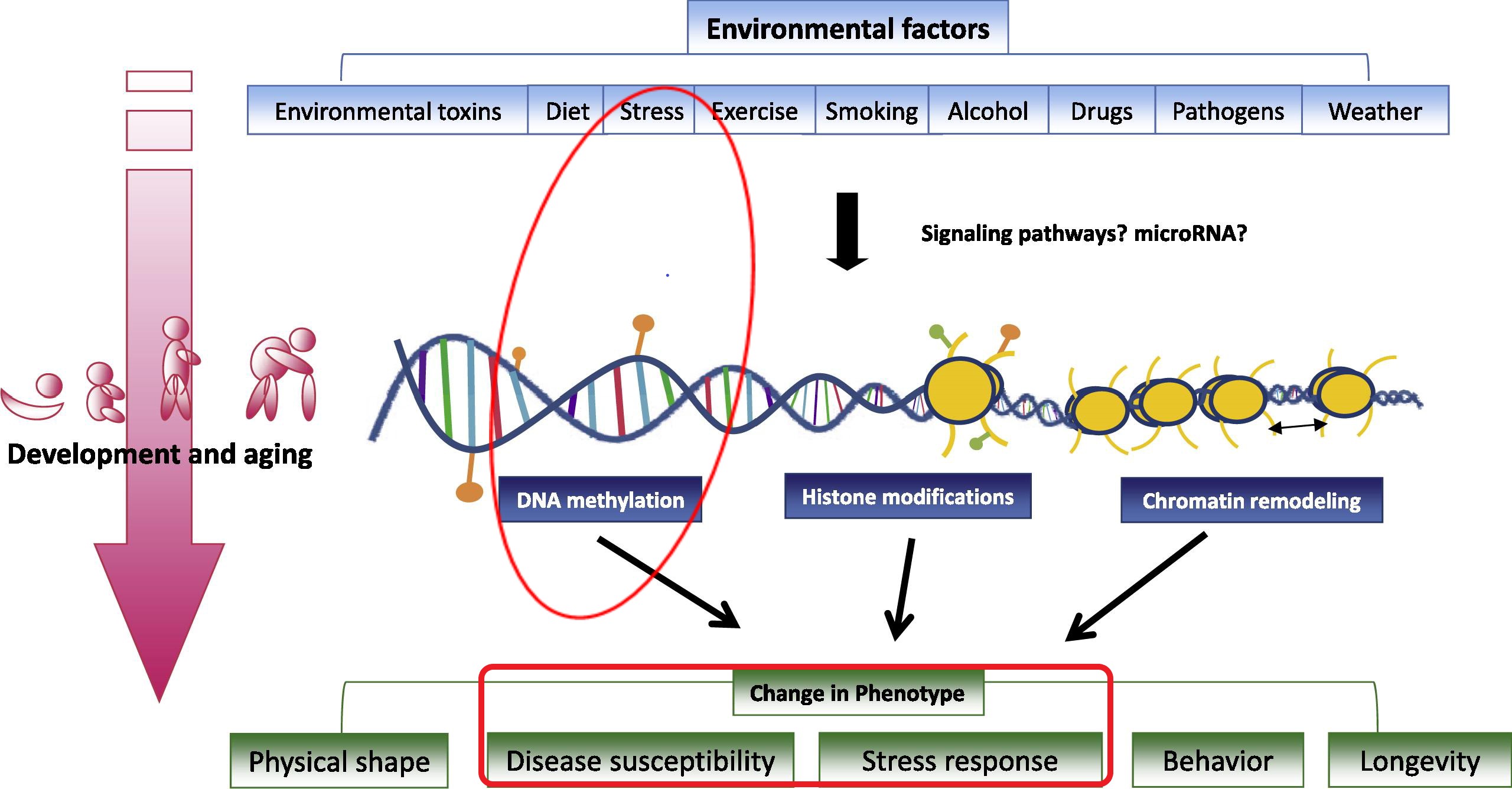
How stressful environment and (epi)genetics can shape phenotype
(adapted from Tammen el al., Mol Aspects Med 2013, 34:753)
Major Achievements
The originality of our program of research lies in its simultaneous examination of the neuropsychiatric consequences of lifetime traumatic events along with physiological stress markers (e.g. diurnal patterns of cortisol secretion, allostatic load), gender specificities, genetic vulnerability, and gene-environment interactions (epigenetics). We notably designed one of the largest epidemiological projects in an elderly cohort (ESPRIT) demonstrating associations between the methylation of promoter regions of specific genes and depression risk (serotonin transporter, BDNF, IL-6,) or Hypothalamic-Pituitary-Adrenal (HPA) stress axis dysfunction (ACE, KITLG). Some associations vary with the genotype, which could reflect endocrinal phenotype heterogeneity and specific resilience capacity. These epigenetic markers could constitute stable peripheral markers of neuropsychiatric disorders or stress reactivity. Within a middle-aged cohort at high risk of developing later life neurodegenerative disorders (the PREVENT project), we constructed a novel data-based model of the interaction between early life trauma, depression, hippocampal volume and dementia risk. Within this project, we are also currently tracking COVID-19 exposure and infection in relation to brain changes (MRI) and stress biomarkers given the vulnerability of limbic system structures to the virus and epidemic associated-environmental stressors.
Ongoing investigations and expected outcomes
- Identification of new pathophysiological stress-related biomarkers at prodromal and preclinical stages and potential endophenotypes between genetic factors and disease phenotypes.
- Better understanding of the mechanisms of resilience.
- Identification of targets for innovative tailor-made preventive and early intervention strategies with possible validation within clinical studies.
Methods
- Large epidemiological studies (ESPRIT, PHOENIX, PREVENT, 13 Novembre), having extensive phenotyping (clinical examinations, MRI, stress evaluation, biobanks), and complementary in terms of age, comorbidities, trauma and age at exposure.
- Physiological (cortisol, catecholamines, metabolic markers, inflammation, sleep) and neuropsychological markers of stress (MRI, fMRI, cognitive functioning, mental flexibility), validated measures of stress exposure (lifetime trauma, posttraumatic stress disorder, social/family support, and resilience), genetic vulnerability and epigenetic modifications (DNA methylation) and other biomarkers (amyloid, tau, ApoE).
- Statistical modelling of censored data, longitudinal data with lifetime exposures and high-dimensional data (brain imaging, genetics…).
Major publications
Norton J, et al. Psychol Med. Feb 10:1-9, 2021
Ritchie K, Carrière I et al., J Neurol Neurosurg Psychiatry. Oct 21, doi: 10.1136/jnnp-2020-323823, 2020
Ancelin ML et al., Depress Anxiety. 37(2):146-155, 2020
Carles S, Carrière I et al., Alzheimers Dement. Sep 3. doi: 10.1002/alz.12149, 2020
Johnson J et al., Psychoneuroendocrinology. 111:104492, 2020
Ancelin ML et al., J Psychiatry Neurosci. 44(1):45-53, 2019
Norton J, et al. Transl Psychiatry. Nov 11;9(1):291, 2019
Ancelin ML & Ryan J, Mol Psychiatry. 23(11):2116-2117, 2018
Carrière I et al., BMC Med. 15(1):81, 2017
Ancelin ML et al., Neurobiol Stress. 7:38-46, 2017
Ritchie K, Carrière I et al., Alzheimers Dement. 13(10):1089-97, 2017
Gandubert C et al., Neurobiol Stress. 3:61-67, 2016
Ryan J et al., Epigenomics. 8(11):1553-1569, 2016 [Invited review]
Januar V et al., Transl Psychiatry. 5:e619, 2015
Ancelin ML et al., Transl Psychiatry. 5:e499, 2015
Chaudieu I et al., J Clin Psychiatry. 72(7):929-935, 2011
Ritchie K et al., BMJ. 341:c3885, 2010
Davydov DM et al., Clin Psychol Rev. 30(5):479-495, 2010
Collaborations
Francis Eustache & Mickaël Laisney (Inserm UMR1077, Cyceron, Caen, France)
Joanne Ryan & Jerome Maller (Monash University, Australia)
Craig Ritchie (University of Edinburgh, UK)
John O’Brien (University of Cambridge, UK)
Dennis Chan (University College London, UK)
International networks & Consortiums (Alzheimer Cohorts Consortium, APPLE Tree, CHARGE, COSMIC, E3, IGAP, Interlace, Lancet Commission on Dementia, TriBEKa)
Fundings
National Health & Medical Research Council (Australia), University of New South Wales/ National Institute of Aging (Australia), NIH (USA), Horizon 2020-IMI, University College London, Alzheimer’s Association UK
Contact